The amount of CO2 released from combustion of fossil fuels, land use changes (i.e. deforestation, agriculture), and concrete production, as opposed to natural ocean and land CO2 sinks, is fairly well known and the atmospheric CO2 increase is in quantitative agreement with the anthropogenic sources minus natural sinks (Le Quéré et al., 2016). On the short time scale since atmospheric CO2 concentrations started increasing beyond the typical Pleistocene CO2 values known from ice cores (i.e. 280 ppm, Bereiter et al., 2015), only major and extended volcanic eruptions could release a similar amount of CO2, but no eruptions have occurred that could explain the magnitude and continuous rise of atmospheric CO2.
In addition to these quantitative assessments, atmospheric CO2 has an unusual geochemical signature that links it to an organic carbon source. Specifically, plants and algae prefer the lighter carbon isotope 12C over the heavier isotopes 13C and 14C, and plant material ends up being isotopically lighter (i.e. enriched in 12C over 13C and 14C; note that 14C is radioactive and carbon molecules ~50,000 years of age are radiocarbon-dead, i.e. they no longer contain any 14C). Because trees and algae form the basic food source for animals and bacteria, and the organic matter of plants, algae and other organisms accumulates in the geological record to form coal, oil and gas, the isotopic signature of fossil fuels is also relatively poor in 13C and devoid of 14C, but rich in 12C. Burning 12C-rich fossil fuels therefore adds a disproportionate amount of 12C to the atmosphere, and this signal can be traced instrumentally today, but also in ancient air trapped in ice cores and in the carbon isotopic composition of marine and terrestrial fossils. This so-called Suess effect (Keeling, 1979) shows that increasing amounts of fossil fuel burning since the industrial revolution have contributed more and more 12C to atmospheric CO2 and the isotopic composition of atmospheric CO2 (i.e. δ13CCO2) thereby documents the organic carbon origin of the ongoing CO2 increase. Similarly, the oxygen concentration in the atmosphere decreases as more and more (fossil) organic carbon is burned (i.e. oxidized), confirming that CO2 is being emitted by oxidation of reduced (i.e. organic) carbon. Finally, there is also a strong correlation between rising CO2 concentrations and the world population since at least the Industrial Revolution (i.e. since the 1850s), supporting the causal relationship determined isotopically and quantitatively from observations of natural and anthropogenic CO2 sources and sinks described above.
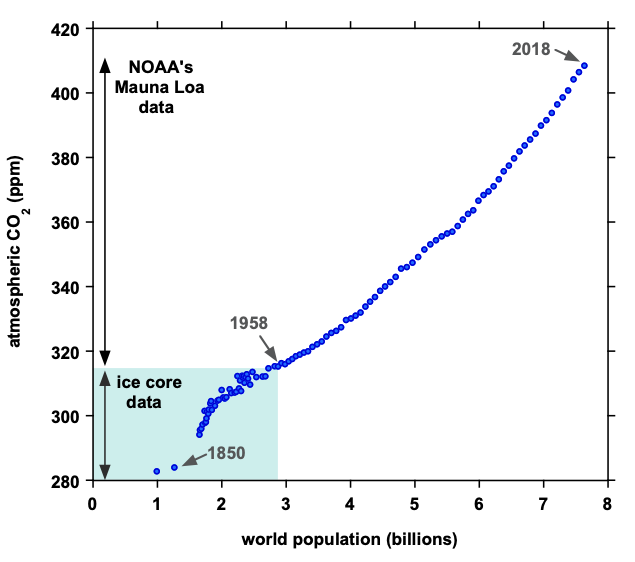 Figure caption:
Figure caption: Atmospheric CO
2 and world population are tightly correlated – more people on the planet emit progressively more CO
2. Data points represent individual years in sequential order, with the first (lowest) presenting the year 1800 and the last (highest) presenting 2018. Data sources are:
world population and
mean annual CO2 from 1959-2018, and Bereiter et al. (ice core CO
2 data highlighted with turquoise box for the years 1800-1958, 2015).
References cited
Bereiter, B., Eggleston, S., Schmitt, J., Nehrbass-Ahles, C., Stocker, T.F., Fischer, H., Kipfstuhl, S. and Chappellaz, J. (2015) Revision of the EPICA Dome C CO2 record from 800 to 600 kyr before present. Geophysical Research Letters 42, 542-549.
Keeling, C.D. (1979) The Suess effect: 13 carbon-14 carbon interrelations. Environment International 2, 229-300.
Le Quéré, C., Andrew, R.M., Canadell, J.G., Sitch, S., Korsbakken, J.I., Peters, G.P., Manning, A.C., Boden, T.A., Tans, P.P., Houghton, R.A., Keeling, R.F., Alin, S., Andrews, O.D., Anthoni, P., Barbero, L., Bopp, L., Chevallier, F., Chini, L.P., Ciais, P., Currie, K., Delire, C., Doney, S.C., Friedlingstein, P., Gkritzalis, T., Harris, I., Hauck, J., Haverd, V., Hoppema, M., Klein Goldewijk, K., Jain, A.K., Kato, E., Körtzinger, A., Landschützer, P., Lefèvre, N., Lenton, A., Lienert, S., Lombardozzi, D., Melton, J.R., Metzl, N., Millero, F., Monteiro, P.M.S., Munro, D.R., Nabel, J.E.M.S., Nakaoka, S.I., O'Brien, K., Olsen, A., Omar, A.M., Ono, T., Pierrot, D., Poulter, B., Rödenbeck, C., Salisbury, J., Schuster, U., Schwinger, J., Séférian, R., Skjelvan, I., Stocker, B.D., Sutton, A.J., Takahashi, T., Tian, H., Tilbrook, B., van der Laan-Luijkx, I.T., van der Werf, G.R., Viovy, N., Walker, A.P., Wiltshire, A.J. and Zaehle, S. (2016) Global Carbon Budget 2016. Earth Syst. Sci. Data 8, 605-649.
 Figure caption: Atmospheric CO2 and world population are tightly correlated – more people on the planet emit progressively more CO2. Data points represent individual years in sequential order, with the first (lowest) presenting the year 1800 and the last (highest) presenting 2018. Data sources are: world population and mean annual CO2 from 1959-2018, and Bereiter et al. (ice core CO2 data highlighted with turquoise box for the years 1800-1958, 2015).
Figure caption: Atmospheric CO2 and world population are tightly correlated – more people on the planet emit progressively more CO2. Data points represent individual years in sequential order, with the first (lowest) presenting the year 1800 and the last (highest) presenting 2018. Data sources are: world population and mean annual CO2 from 1959-2018, and Bereiter et al. (ice core CO2 data highlighted with turquoise box for the years 1800-1958, 2015).